|
Here, the scientists reveal an unexpected role for the lysosomal protein prosaposin (PSAP), the knockdown of which caused the formation of lipofuscin, a hallmark of aging, which traps iron, generating reactive oxygen species and triggering ferroptosis. Intriguingly, PSAP deficiency caused these dramatic phenotypes only in neurons, but not in other cells. Learn how the authors used Dojindo's Ferroptosis-related products, FerroOrange and Liperfluo for detecting Iron levels and lipid peroxidation, in their study.
|
|
Genome-wide CRISPRi/a screens in human neurons link lysosomal failure to ferroptosis Ruilin Tian, et. al., Nature Neuroscience (2022)
Point of Interest
- The study reveals pathways that govern neuronal response to chronic oxidative stress, a factor in neurodegenerative diseases.
- Suppression of the lysosomal protein prosaposin makes neurons highly susceptible to oxidative stress.
- This happens through the induction of lipofuscin formation which sequesters iron.
- Iron accumulation contributes to creating reactive oxygen species and lipid peroxidation, triggering ferroptosis, exclusively in neurons.
|
|
Related Techniques
|
- Intracellular lipid peroxidation measurement
- Liperfluo
|
- Mitochondria lipid peroxidation measurement
- MitoPeDPP
|
- Intracellular ferrous ion (Fe2+) detection
- FerroOrange
|
- Mitochondria ferrous ion (Fe2+) detection
- Mito-FerroGreen
|
- Mitochondrial superoxide detection
- MitoBright ROS Deep Red - Mitochondrial Superoxide Detection
|
- Lysosomal function assay
- Lysosomal pH and mass detection Kit
|
- Cellular senescence detection (Live cell imaging or FCM)
- Cellular Senescence Detection Kit
|
- Cellular senescence detection (Plate reader)
- Cellular Senescence Plate Assay Kit
|
|
Related Applications
|
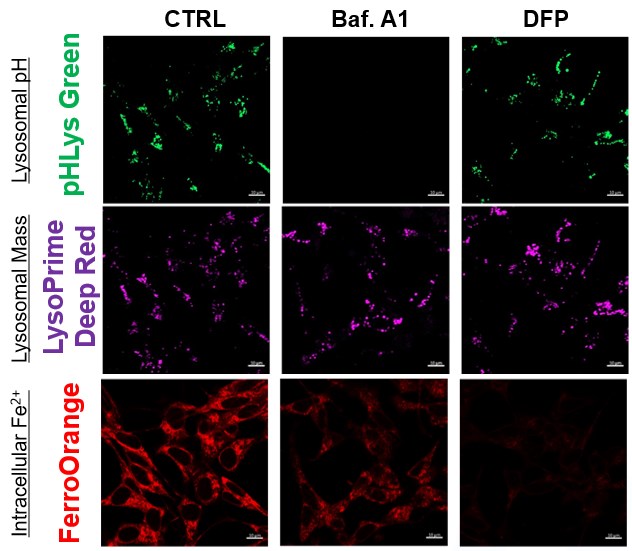
The simultaneous detection of lysosomal function with Mitochondrial ROS and intracellular Fe2+
Lysosomal Function and Iron Homeostasis
-
Recent reports suggest that lysosomal neutralization can result in iron depletion, consequently leading to the disruption of cell viability. To verify this, HeLa cells were labeled with FerroOrange for Fe2+ detection, and the lysosomal mass and pH were separately detected with LysoPrime DeepRed and pHLys Green (a product currently under development). Co-staining with FerroOrange and Lysosomal dyes demonstrated that Bafilomycin A1 (Baf. A1), an inhibitor of lysosomal acidification, causes iron depletion consistent with the findings reported in the article. Interestingly, the iron chelator, Deferiprone (DFP), did not impact lysosomal pH, suggesting that lysosomal function plays a key role in managing iron homeostasis.
Reference: Ross A Weber, et. al., Mol Cell (2020)
Products in Use
- FerroOrange
- pHLys Green*
- LysoPrime Deep Red
*pHLys Green will be available in July 2023 as the "Lysosomal Acidic pH Detection Kit-Green/Deep Red". If you would like to receive the promotional informaiton, please click here and write "New Product Information" in the inquiry box.
|
|
Lysosomal Function and Mitochondrial ROS
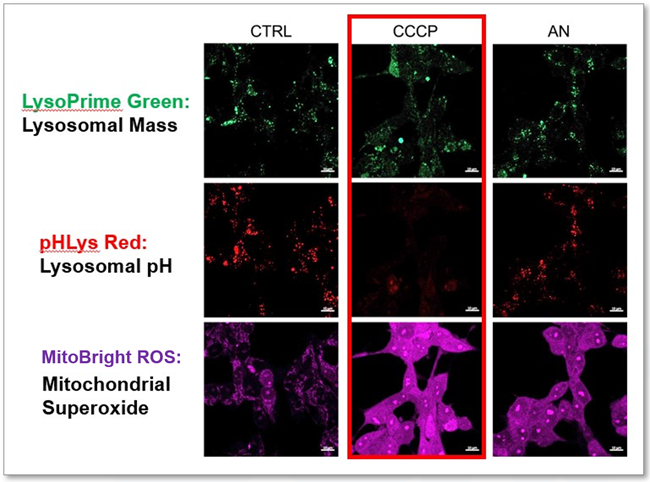
-
CCCP and Antimycin are recognized inducers of mitochondrial ROS, linked to the loss of mitochondrial membrane potential. Recent studies have shown that CCCP induces not only mitochondrial ROS but also lysosomal dysfunction. To observe mitochondrial ROS, HeLa cells were labeled with MitoBright ROS Deep Red for Mitochondrial Superoxide Detection, and the lysosomal mass and pH were independently detected with LysoPrime Green and pHLys Red. Co-staining with MItoBright ROS and Lysosomal dyes revealed that CCCP, unlike Antimycin, triggers concurrent lysosomal neutralization and mitochondrial ROS induction.
Reference: Benjamin S Padman, et. al., Autophagy (2013)
Products in Use
- LysoPrime Green
- pHLys Red
- Lysosomal Acidic pH Detection Kit
- MitoBright ROS Deep Red - Mitochondrial Superoxide Detection
|
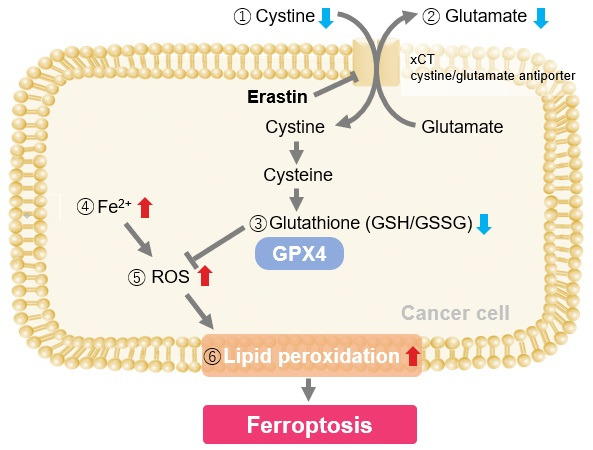
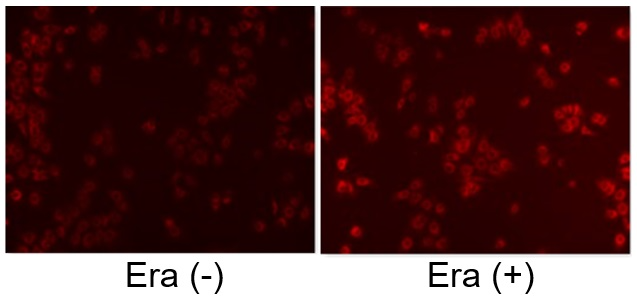
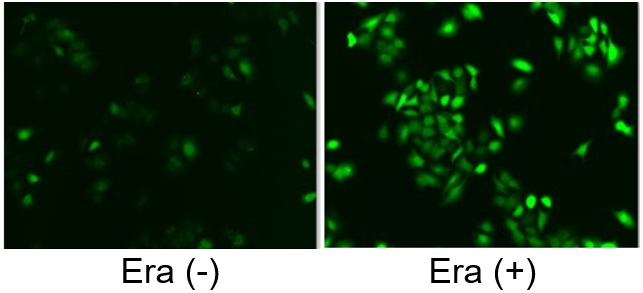
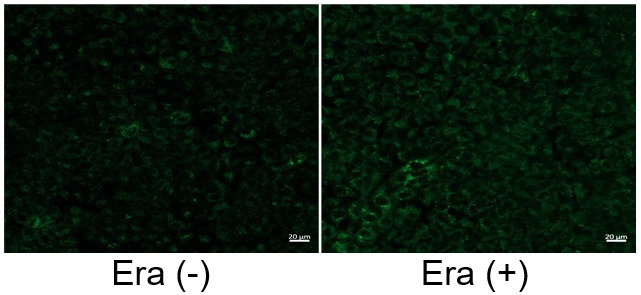
Induction of Ferroptosis by Erastin
Erastin is a known inducer of ferroptosis. By inhibiting the cystine transporter (xCT), erastin inhibits the uptake of cystine. Cystine is the raw material for GSH. Therefore, Erastin ultimately decreases the amount of GSH. Decreased GSH then results in lipid peroxide accumulation and induction of ferroptosis.
The following experimental examples show changes in each aforementioned index as a consequence of erastin stimulation. Measurements are made using Dojindo reagents.
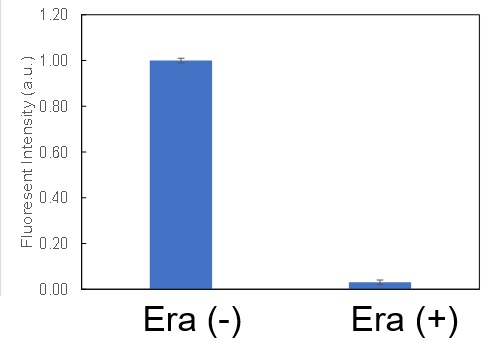
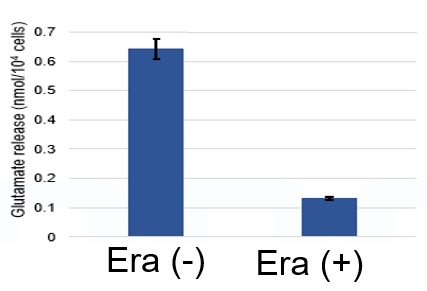
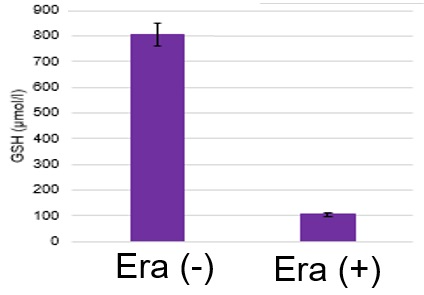
Using erastin-treated A549 cells, we measured intracellular Fe2+, ROS, lipid peroxide, glutathione, glutamate release into the extracellular space, and cystine uptake. As a result, inhibition of xCT by elastin was observed and also the release of glutamate and uptake of cystine were decreased. Furthermore, elastin treatment decreased intracellular glutathione while it increased intracellular Fe2+ , ROS, and lipid peroxides.
|