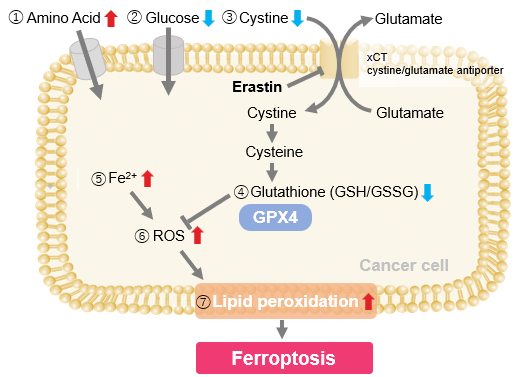
| GPX4 is a central suppressor of ferroptosis that reduces peroxidized phospholipids within cellular membranes, and its regulation is closely linked to the prevention of neurodegenerative diseases and the development of cancer therapies. Accordingly, new insights into GPX4 biology are important for advancing our understanding of ferroptosis control. Recent studies have shown that mutations disrupting the membrane-anchoring mechanism of GPX4 cause its mislocalization, which is sufficient to induce ferroptosis and neurodegeneration independently of enzymatic activity. In addition, PRDX6 has been identified as a selenium-acceptor protein required for GPX4 synthesis, and loss of PRDX6 leads to reduced GPX4 abundance and increased sensitivity to ferroptosis. Together, these findings provide an important foundation for modulating ferroptosis through GPX4 regulation and translating this knowledge into therapeutic applications. | ||||||||||||||||||||||
|
1. A fin-loop-like structure in GPX4 underlies neuroprotection from ferroptosis (Cell, 2025) Highlighted technique: To evaluate the importance of GPX4 localization, patient-derived primary fibroblasts carrying the R152H mutation in the membrane-anchoring fin-loop of GPX4 were used to analyze the impact of anchor disruption. Lipid peroxidation was assessed using BODIPY 581/591 C11, demonstrating that the R152H mutant exhibits increased accumulation of peroxidized lipids. |
||||||||||||||||||||||
|
2. PRDX6 dictates ferroptosis sensitivity by directing cellular selenium utilization (Molecular Cell, 2024) Highlighted technique: Using PRDX6 KO cells, this study shows that loss of PRDX6 markedly reduces GPX4 protein levels without altering GPX4 mRNA expression, demonstrating that PRDX6 is required for GPX4 synthesis rather than transcription. Consistently,PRDX6-deficient mice exhibit a selective reduction of GPX4 expression in the brain, where selenium supply and utilization are physiologically constrained, highlighting the essential role of PRDX6 in maintaining GPX4 abundance under selenium-limited conditions. |
||||||||||||||||||||||
Ferroptosis Indicators (click to open/close)
|
||||||||||||||||||||||
Application Note (click to open/close)
|
||||||||||||||||||||||
|
|
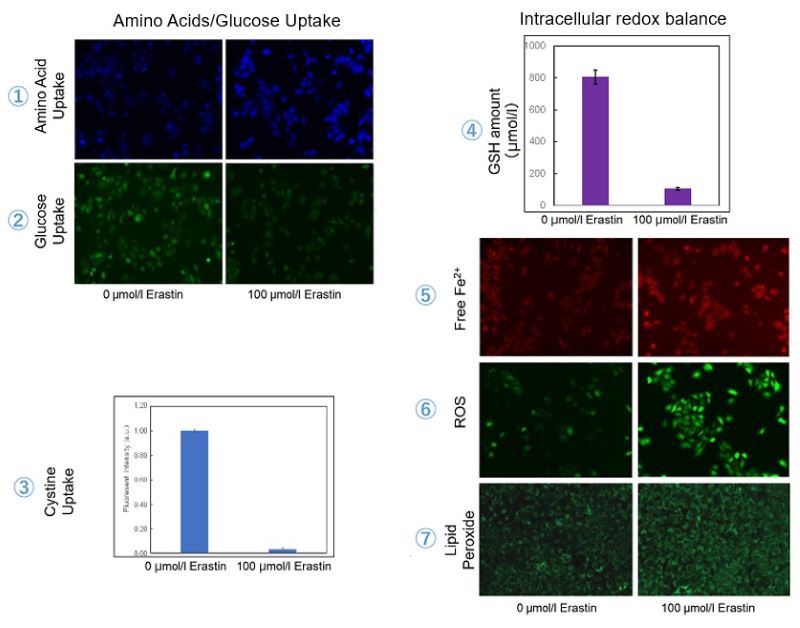
We investigated the transition of cellular metabolisms in A549 cells treated with erastin, a known ferroptosis inducer. Our results revealed the following. Results Cell Line: A549 |
||
|
Products in Use |
 |
||