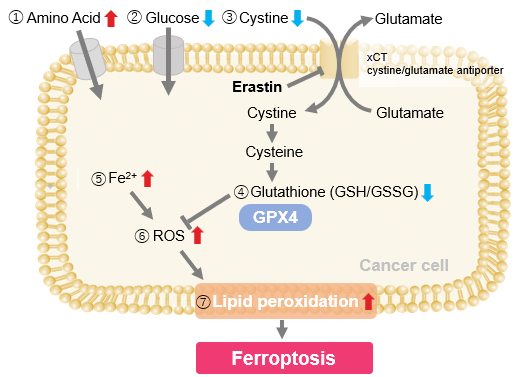
| Ferroptosis is an iron-dependent form of cell death shaped by lipid peroxidation as well as intracellular factors such as iron localization and lysosomal acidity. Lysosomes function as central sites for iron storage and mobilization, and emerging evidence suggests that iron activation and lipid peroxidation within lysosomes contribute to ferroptosis induction. Recent studies show that BDH2-dependent iron transfer between mitochondria and lysosomes in melanoma prevents Fe(II) sequestration, whose loss increases ferroptosis vulnerability. Another study demonstrates that lysosomal alkalinization during senescence causes abnormal Fe(II) retention, forming a major basis of ferroptosis resistance. These findings highlight lysosome-centered iron regulation as a key determinant of ferroptosis sensitivity. | ||||||||||||||||||||||
|
1. BDH2-driven lysosome-to-mitochondria iron transfer shapes ferroptosis vulnerability of the melanoma cell states (Nature Metabolism, 2025) Related technique Intracellular Fe2+ Detection, Mitochondrial Fe2+ Detection |
||||||||||||||||||||||
|
2. Senescence-associated lysosomal dysfunction impairs cystine deprivation-induced lipid peroxidation and ferroptosis (Nature Communications, 2025) Related technique Lysosomal Fe2+ Detection, Lysosome function Analysis |
||||||||||||||||||||||
Ferroptosis Indicators (click to open/close)
|
||||||||||||||||||||||
Application Note (click to open/close)
|
||||||||||||||||||||||
|
|
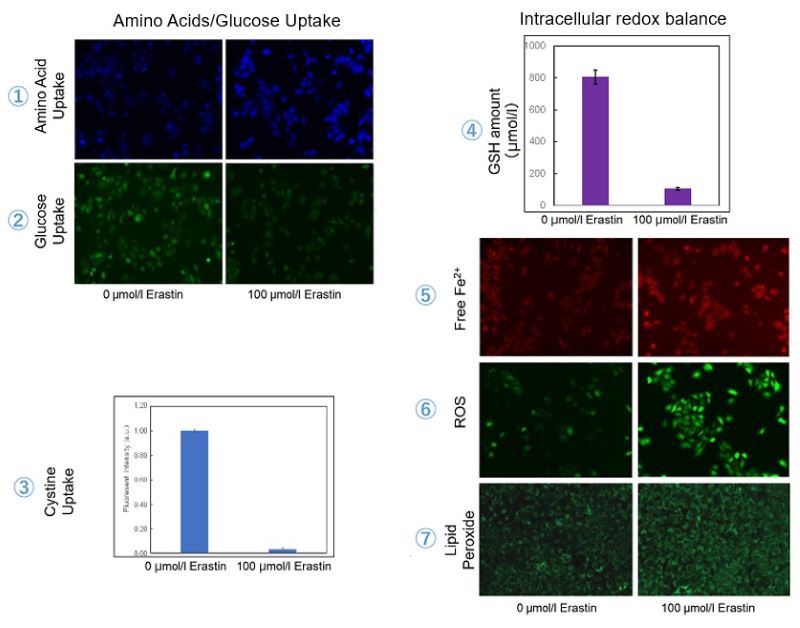
We investigated the transition of cellular metabolisms in A549 cells treated with erastin, a known ferroptosis inducer. Our results revealed the following. Results Cell Line: A549 |
||
|
Products in Use |
 |
||