| Recently, the importance of lysosomal iron in ferroptosis induction has attracted increasing attention. Recent studies in melanoma have identified a protein involved in iron flux at mitochondria–lysosome contact sites, revealing that organelle-to-organelle iron compartmentalization plays a role in ferroptosis regulation. In addition, research on senescent cells has highlighted that, despite iron accumulation, ferroptosis resistance is linked to lysosomal pH, demonstrating that lysosomal acidity is another key determinant of ferroptosis sensitivity. Together, these studies deepen our understanding of how the lysosomal environment contributes to ferroptosis regulation. | ||||||||||||||||||||||||
|
1. BDH2-driven lysosome-to-mitochondria iron transfer shapes ferroptosis vulnerability of the melanoma cell states (Nature Metabolism, 2025) Highlighted technique: To compare organelle iron distribution between melanoma cell states, the authors stained cells with FerroOrange for ferrous iron and lysosome detection probes. They observed increased overlap of both dyes in mesenchymal-like cells, indicating iron enrichment at mitochondria–lysosome contact regions during the phenotype transition. Related technique Intracellular Fe2+ detection (as used in this article), pH-Insensitive Lysosome Detection |
||||||||||||||||||||||||
|
2. Senescence-associated lysosomal dysfunction impairs cystine deprivation-induced lipid peroxidation and ferroptosis (Nature Communications, 2025) Highlighted technique: To test whether restoring lysosomal acidity can reverse ferroptosis resistance in senescent cells, EN6 was used to activate V-ATPase and reacidify lysosomes. Lysosomal pH, lysosomal ferrous ions, and lipid peroxidation were visualized, revealing that acidity restoration released trapped iron within lysosomes and reactivated ferroptosis. Related technique Lysosome Acidic pH Detection, Cellular Senescence Detection |
||||||||||||||||||||||||
Related Techniques (click to open/close)
|
||||||||||||||||||||||||
Application Note (click to open/close)
|
||||||||||||||||||||||||
|
|
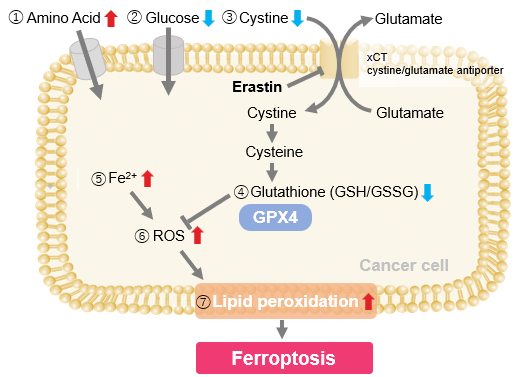
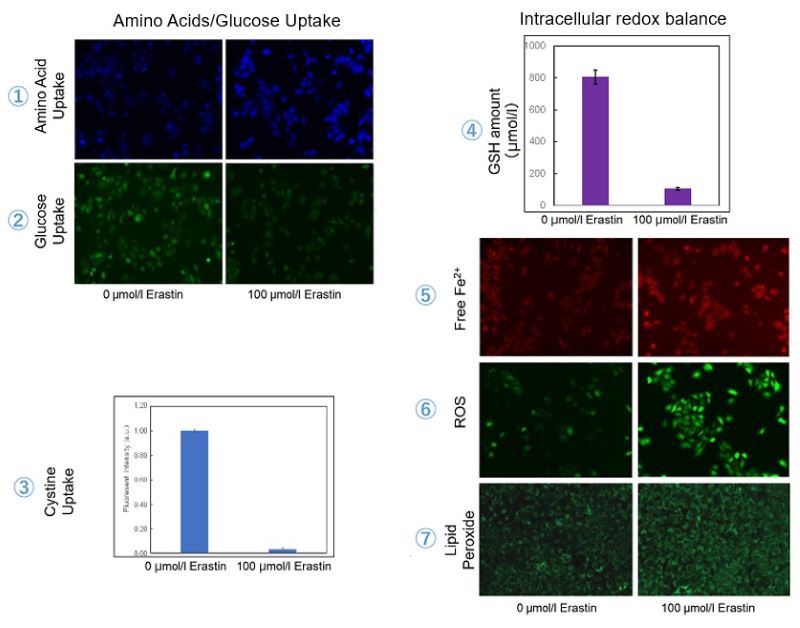
We investigated the transition of cellular metabolisms in A549 cells treated with erastin, a known ferroptosis inducer. Our results revealed the following. Results Cell Line: A549 |
||
|
Products in Use |
 |
||