| |
Cell
|
Senescence induction
|
Senescence marker(s)
|
Responding variable(s)
|
Reference
|
| ① |
IMR90
(Human pulmonary fibroblasts)
|
Several passages in culture
|
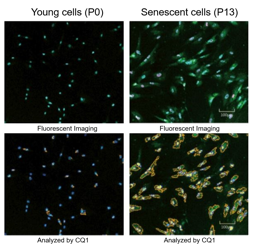
SA-ß-Gal, p16, p21, Nucleosome hypertrophy
|
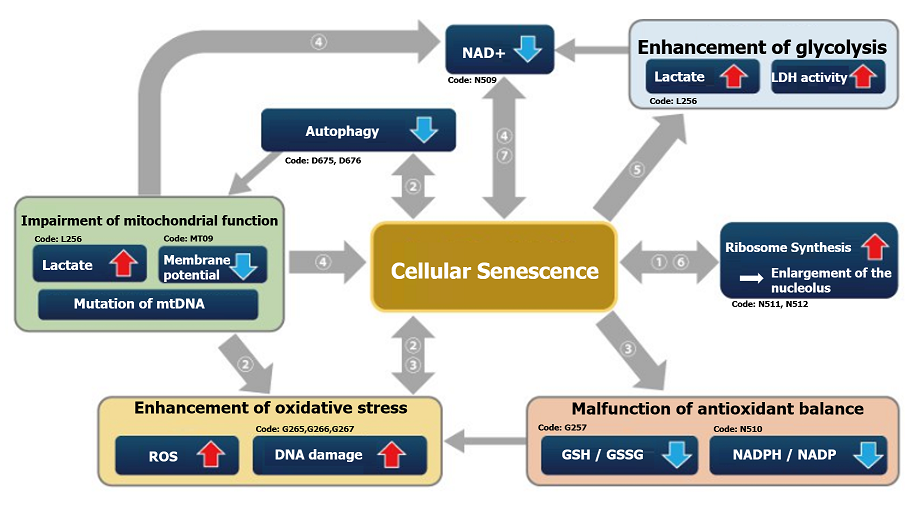
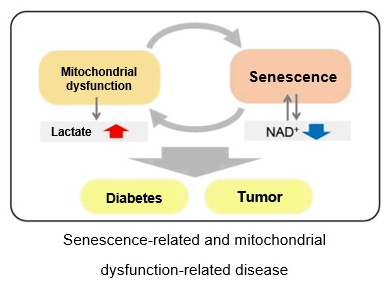
Expression of SETD8↓, H4K20me1↓, oxidative phosphorylation↑, ribosome synthesis↑
|
H. Tanaka, S. Takebayashi, A. Sakamoto, N. Saitoh, S. Hino, and M. Nakao, “The SETD8/PR-Set7 Methyltransferase Functions as a Barrier to Prevent Senescence-Associated Metabolic Remodeling.”, Cell Reports, 2017, 18(9), 2148.
|
|
Inhibition of SETD8
(Methyltransferase)
|
Oxidative phosphorylation↑, ribosome synthesis↑
|
| ② |
Senescent mouse satellite cell
eletal muscle progenitor cells)
|
-
|
SA-β-Gal, p16
|
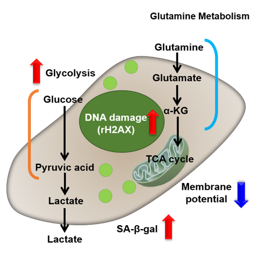
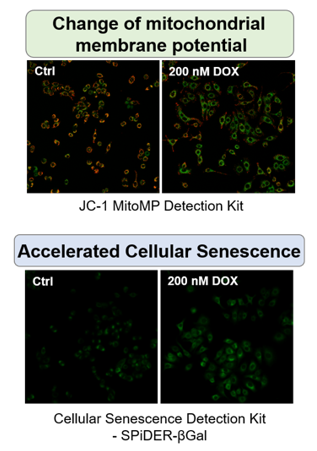
Autophagy activity↓, ROS↑, mitochondrial membrane potential↓
|
L. Garcia-Prat, M. Martinez-Vicente, and P. Munoz-Canoves, “Autophagy: a decisive process for stemness”, Oncotarget, 2016, 7(11), 12286.
|
|
Atg7 knockout mouse
(Satellite cells)
|
Autophagy inhibition
|
SA-ß-Gal, P15, p16, p21, γ-H2AX
|
ROS↑, mitochondrial membrane potential↓
|
| ③ |
Rat fibroblast model of type 2 diabetes
|
-
|
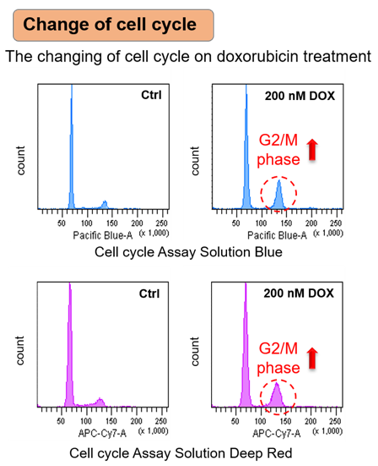
SA-ß-Gal, p21, p53, γ-H2AX
|
NADP+/ NADPH↓(resistance to oxidative stress↓), NADPH oxidase↑(ROS↑)
|
M. Bitar, S. Abdel-Halim and F. Al-Mulla, “Caveolin-1/PTRF upregulation constitutes a mechanism for mediating p53-induced cellular senescence: implications for evidence-based therapy of delayed wound healing in diabetes”, Am J Physiol Endocrinol Metab.”, 2013, 305(8), E951.
|
| ④ |
IMR90
(Human pulmonary fibroblasts)
|
Ethidium bromide (inhibition of mtDNA) + pyruvate deficiency
|
SA-ß-Gal
|
NAD+ / NADH ↓
|
C. Wiley, M. Velarde, P. Lecot, A. Gerencser, E. Verdin, J. Campisi, et. al., “Mitochondrial Dysfunction Induces Senescence with a Distinct Secretory Phenotype”, Cell Metab., 2016, 23(2), 303.
|
| ⑤ |
MDA-MB-231
(Human breast cancer cells)
|
X-ray irradiation + inhibition of cell cycle-related factor (securin) expression
|
SA-ß-Gal
|
Lactate↑, LDH activity↑, (glycolysis↑)
|
E. Liao, Y. Hsu, Q. Chuah, Y. Lee, J. Hu, T. Huang, P-M Yang & S-J Chiu, “Radiation induces senescence and a bystander effect through metabolic alterations.”, Cell Death Dis., 2014, 5, e1255.
|
| ⑥ |
MEF
(Mouse Embryonic Fibroblast)
|
Overexpression of oncogenes,several passages in culture, transcription factor overexpression(E2F1)
|
SA-ß-Gal, p16, p21, Nucleosome hypertrophy
|
Ribosome RNA↑,p53↑
|
K. Nishimura, T. Kumazawa, T. Kuroda, A. Murayama, J. Yanagisawa and K. Kimura, “Perturbation of Ribosome Biogenesis Drives Cells into Senescence through 5S RNP-Mediated p53 Activation”, Cell Rep. 2015, 10(8), 1310.
|
| ⑦ |
Mouse tail fibroblast
|
2 months old, 22 months old, p16 knockout (22 months old)
|
SA-ß-Gal, p14, p16
|
NAD+ ↓、SIRT3 ↓
|
M. J. Son, Y. Kwon, T. Son and Y. S. Cho, “Restoration of Mitochondrial NAD+ Levels Delays Stem Cell Senescence and Facilitates Reprogramming of Aged Somatic Cells”, Stem Cells. 2016, 34(12), 2840.
|