|
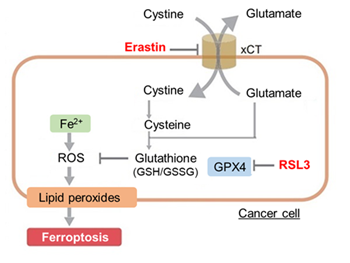
Cell death involves plasma membrane rupture or remodeling and the release of cell derived material into the extracellular space. Extracellular vesicles can serve as carriers for such material, and how death associated membrane features are incorporated into vesicles and influence the local response after cell death is an important mechanistic question. Recent work shows that pyroptotic cells release vesicles bearing preformed gasdermin D pores, and these pores can transfer to neighboring nonpyroptotic cells and trigger lytic membrane disruption. A separate study shows that adherent apoptotic cells leave actin rich remnants that later round up into large phosphatidylserine positive vesicles that are engulfed by macrophages. Together, these findings indicate that membrane features generated during cell death can be externalized via extracellular vesicles and linked to the local response after cell death. |
||||||||||||||||||||||||||
|
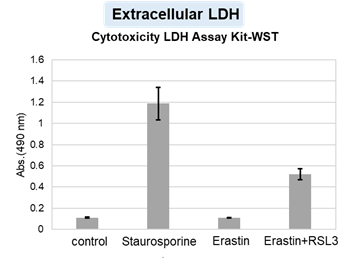
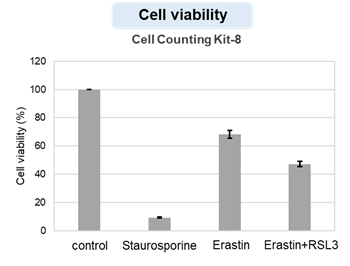
Summary: Pyroptosis is an inflammatory form of cell death in which gasdermin D (GSDMD) forms pores in the plasma membrane, leading to membrane rupture. In this study, pyroptotic cells were shown to release extracellular vesicles (EVs) carrying pre-formed GSDMD pores, which can transplant these pores onto nearby cells not undergoing pyroptosis, causing lytic membrane disruption and suggesting how tissue injury and inflammation may spread in vivo. Highlighted technique: To test whether membrane pores generated during pyroptosis can spread to neighboring cells via EVs, the authors used a transwell system and confirmed that pyroptotic cells can induce cell death in physically separated, non-contact cells. They then isolated EVs from pyroptotic cell supernatants by ultracentrifugation and size-exclusion chromatography, analyzed EV populations containing fluorescently labeled GSDMD by flow cytometry, and confirmed that these EVs can induce cell death in nearby cells. |
||||||||||||||||||||||||||
|
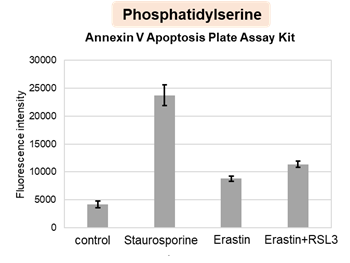
Summary: When adherent cells undergo apoptosis, they leave actin-rich “footprints of death” (FOOD) that remain anchored at the death site and later round up into large extracellular vesicles (EV) exposing phosphatidylserine. By keeping an “eat-me” signal fixed at the site of cell death and generating vesicles that are engulfed by macrophages, FOOD can act as a local flag that promotes clearance of apoptotic debris. Highlighted technique: To observe the formation of “footprints of death” (FOOD) and their conversion into FOOD-derived apoptotic extracellular vesicles (F-ApoEV), the authors performed live-cell 3D time-lapse imaging of apoptotic adherent cells after membrane labeling, while monitoring phosphatidylserine exposure with Annexin V and visualizing nuclei. This approach allowed them to track flat FOOD structures that remain beneath cells as they shrink and detach, and to follow their local rounding on the surface into large vesicles (F-ApoEV) at the site of cell death. |
||||||||||||||||||||||||||
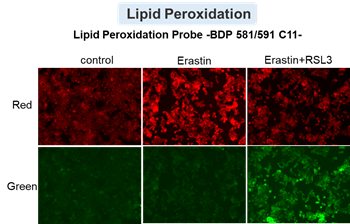
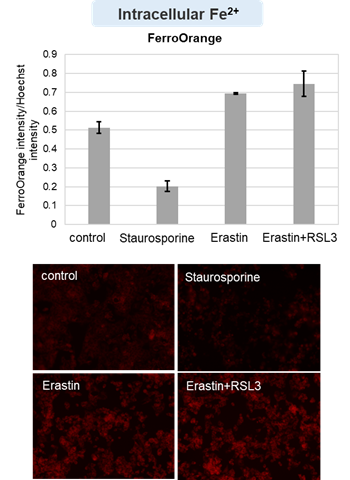
Cell Death Indicators (click to open/close)
|
||||||||||||||||||||||||||
Application Note (click to open/close)
|
||||||||||||||||||||||||||