Previous Science Note
|
ATP release during cell death plays a central role in immune activation, but different cell death modalities either suppress, delay or enhance this release through different molecular mechanisms. This Science Note introduces recent studies that shed light on how different cell death pathways regulate ATP dynamics and influence immunological outcomes. |
||||||||||||||||||||
|
Summary: While cell death typically involves the release of DAMPs such as ATP, which trigger immune responses, certain forms of apoptosis suppress this release. This study shows that ATP is sequestered via an unconventional autophagic pathway, thereby limiting its extracellular release and maintaining the immunosilent nature of apoptosis. Highlighted technique: This study showed that blocking the BAK-dependent non-canonical autophagy pathway during apoptosis increased extracellular ATP and reduced ATP in apoptotic bodies. Co-culture with ATP and these apoptotic bodies enhanced IL-1β production by macrophages, suggesting that inhibition of this pathway promotes immune activation. Related technique Apoptosis Plate Assay, Extracellular ATP Assay, Autophagy Flux Detection |
||||||||||||||||||||
|
Summary: Pyroptosis is a form of necrotic cell death induced by cleavage of gasdermin D (GSDMD) and subsequent pore formation by the N-terminal domain. This study shows that at an early stage of GSDMD-mediated plasma membrane permeabilization, ATP is transiently released prior to cell death and IL-1β secretion, acting as an early danger signal. Highlighted technique: To investigate ATP release, the authors used GSDMD-deficient mice and found that extracellular ATP was not released upon NLRP3 activation without GSDMD. In contrast, inhibition of membrane rupture suppressed LDH but not ATP release, suggesting that ATP release is an early event independent of cell lysis. Related technique Extracellular ATP Assay, Released LDH Assay |
||||||||||||||||||||
|
Summary: Some cancer therapies are exploring the use of genetically engineered cancer cells to stimulate the immune system in a safe and controlled manner. This study shows that activating a RIPK3-based cell death system in such cells triggers the release of ATP, which boosts the immune response and improves both the safety and efficacy of cancer cell-based treatments. Highlighted technique: The researchers engineered cancer cells to express a drug-inducible RIPK3 construct and implanted these cells into the brains of mice. When the RIPK3 switch was activated in vivo, necroptosis was induced, leading to the release of ATP and DAMPs, which in turn stimulated anti-tumour immune responses and promoted long-term immune activation. Related technique Extracellular ATP Assay, Apoptosis Plate Assay |
||||||||||||||||||||
Related Techniques (click to open/close)
|
||||||||||||||||||||
Application Note I (click to open/close)
|
||||||||||||||||||||
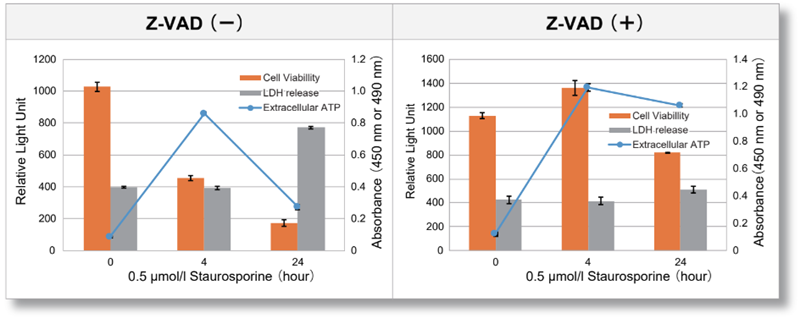
| Jurkat cells were treated with or without Z-VAD, an apoptosis inhibitor, and then treated with staurosporine. After treatment, changes in extracellular ATP release, cell viability and extracellular LDH release were assessed over time. The results showed that cell death was inhibited by Z-VAD, but extracellular ATP released during the initial phase of apoptosis increased over time. | |||
|
|
<Product in use> LDH release Cell viability |
||
Application Note II (click to open/close)
> Ferroptosis Induction and ATP Release Profile
|
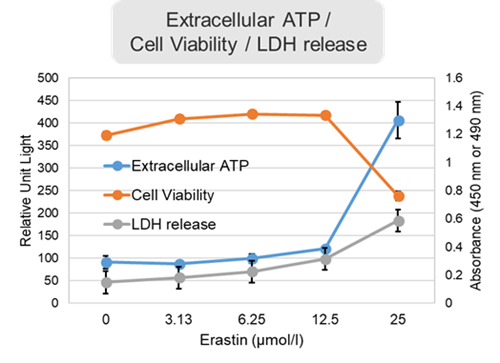
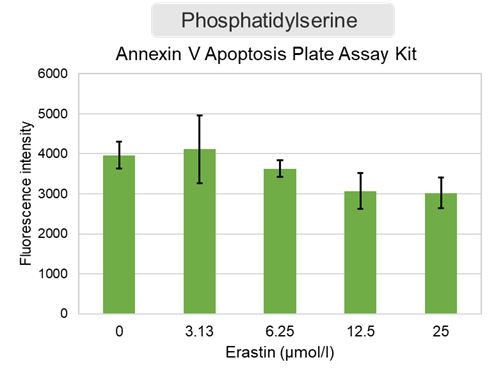
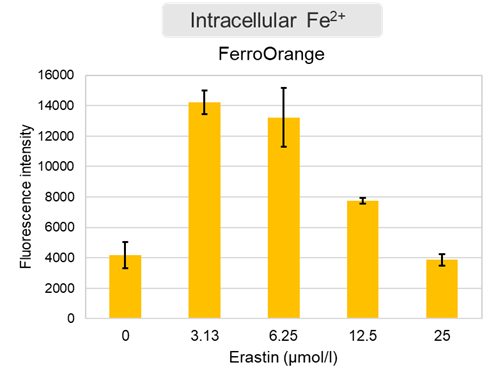
Changes in extracellular ATP release, cell viability, extracellular LDH release, phosphatidylserine, and intracellular Fe2+ were evaluated in HeLa cells treated with various concentrations of Erastin, a ferroptosis inducer, for 24 hours. The results showed that cell viability decreased and extracellular ATP release and extracellular LDH increased in cells treated with Erastin concentration of 25 μmol/l, indicating that cell death was induced under high concentration conditions. Interestingly, the increase in extracellular ATP in the early phase of stimulation, which was observed with the apoptosis inducer Staurosporine, was not observed with Erastin (See Experimental Example: Evaluation using Staurosporine-treated Cells.). Although the apoptosis-related marker phosphatidylserine was not significantly altered by Erastin treatment at any concentration. The amount of intracellular Fe2+, a ferroptosis-related marker, was significantly increased under the low-concentration treatment condition, indicating that it tends to increase before actual cell death occurs. |
<Product in use> Extracellular LDH: Cell viability: Cell Counting Kit-8 Phosphatidylserine: Intracellular Fe2+: FerroOrange |
||
 |
 |
 |
|