|
Proteostasis refers to the cellular mechanisms that regulate the synthesis, folding, trafficking, and degradation of proteins to maintain overall protein balance and health within the cell. Disruptions in proteostasis, such as misfolded proteins or impaired degradation pathways, can lead to protein aggregates that are toxic to cells, a common feature of neurodegenerative diseases such as Alzheimer's and Parkinson's. Maintaining proteostasis is critical in neurons because their long lifespan and high metabolic activity make them particularly susceptible to the accumulation of protein aggregates. Perturbations in proteostasis can initiate or exacerbate neurodegenerative processes, ultimately contributing to neuronal dysfunction and cell death.
|
-
Accumulation of APP C-terminal fragments causes endolysosomal dysfunction through the dysregulation of late endosome to lysosome-ER contact sites
Click here for the original article: Marine Bretou, et. al., Developmental Cell, 2024.
Point of Interest
- Chronic inhibition of γ-secretase leads to a decrease in lysosomal Ca2+ and ultimately to resulting in endolysosomal collapse.
- APP(amyloid precursor protein) C-terminal fragments (APP-CTFs) localize to late endosome/lysosome-ER contacts and an excess of APP-CTFs reduces lysosomal Ca2+ replenishment from the ER.
- Balanced levels of APP-CTF are important for lysosomal homeostasis.
-
NAD depletion mediates cytotoxicity in human neurons with autophagy deficiency
Click here for the original article: Congxin Sun, et. al., Cell Reports, 2023.
Point of Interest
- Loss of autophagy in neurons leads to a metabolic defect and cell death.
- Dysfunctional autophagy causes NAD depletion due to hyperactivation of NAD-consuming enzymes.
- NAD depletion leads to cell death via mitochondrial depolarization in neurons.
- Increasing intracellular NAD levels rescues the viability of autophagy-deficient neurons.
-
Integrative analysis reveals a conserved role for the amyloid precursor protein in proteostasis during aging
Click here for the original article: Nature Communications, et. al., Cell Reports, 2023.
Point of Interest
- Drosophila ortholog of APP, Appl, plays a role in controlling multiple cellular pathways, including translation, mitochondrial function, nucleic acid and lipid metabolism, cellular signaling, and proteostasis in the aging brain.
- Appl regulated autophagy through TGFβ signaling in mouse genetic, human iPSC and in vivo tauopathy models.
- The role of APP in controlling age-dependent proteostasis is conserved and associated with Alzheimer's disease.
|
|
Related Techniques
|
- Lysosomal function
- Lysosomal Acidic pH Detection Kit-Green/Red and Green/Deep Red
|
|
|
- Autophagy detection
- DAPRed (Autophagosome detection), DALGreen (Autolysosome detection)
|
- Mitophagy detection dye
- Mitophagy Detection Kit and Mtphagy Dye
|
- Cellular senescence detection
- SPiDER-βGal for live-cell imaging or flow cytometry / microplate reader / tissue samples.
|
- Glycolysis/Oxidative phosphorylation Assay
- Extracellular OCR Plate Assay Kit, Glycolysis/OXPHOS Assay Kit
|
- Lipid droplet detection
- Lipid Droplet Assay Kit - Blue / Deep Red
|
- NAD(H) and NADP(H) redox couples assay
- NAD/NADH and NADP/NADPH Assay Kit
|
|
Related Applications
|
Tracing autophagosome to autolysosome in live cells
Nampt inhibitor, FK866 inhibits the progress of autophagosome to autolysosome by lysosomal deacidification. A recent finding shows that the dysfunctional condition of nicotinamide adenine dinucleotide (NAD+) biosynthetic enzyme, Nampt induces lysosomal deacidification1). In this section, we tried to determine how NAD+ depletion-induced lysosomal deacidification affects the autophagy-lysosomal pathway. 1) Mikako Yagi, et. al., EMBO J., 40(8), e105268 (2021)
-
-
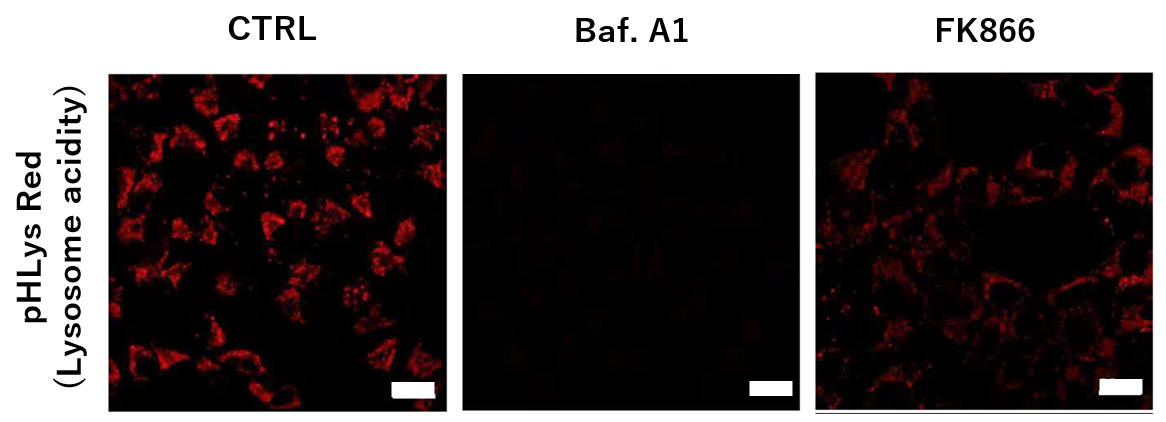
To confirm the effect of the Nampt inhibitor, FK866, on lysosomal acidification, HeLa cells were first labeled by the lysosomal pH detection dye pHLys Red. The cells were then treated with FK866, and lysosomal acidification inhibitor Bafilomycin A1 was used as a positive control. FK866 and Bafilomycin A1-treatment each decreased the fluorescent pHLys Red signal, indicating lysosome neutralization.
(Protocol)
HeLa cells (8 well ibidi) (MEM, FBS+)
↓
Wash x2 (HBSS)
↓
. pHLys Red working solution (HBSS, 1,000 times dilution)
. pHLys Red working solution (HBSS, 1,000 times dilution) + 50 nM Bafilomycin
A1
. pHLys Red working solution (HBSS, 1,000 times dilution) + 10 nM FK866
↓
30 min, 37°C
↓
Observed by confocal microscopy (x20)
|
Simultaneous Detection of Lysosomal and Mitochondrial Dysfunction
-
-
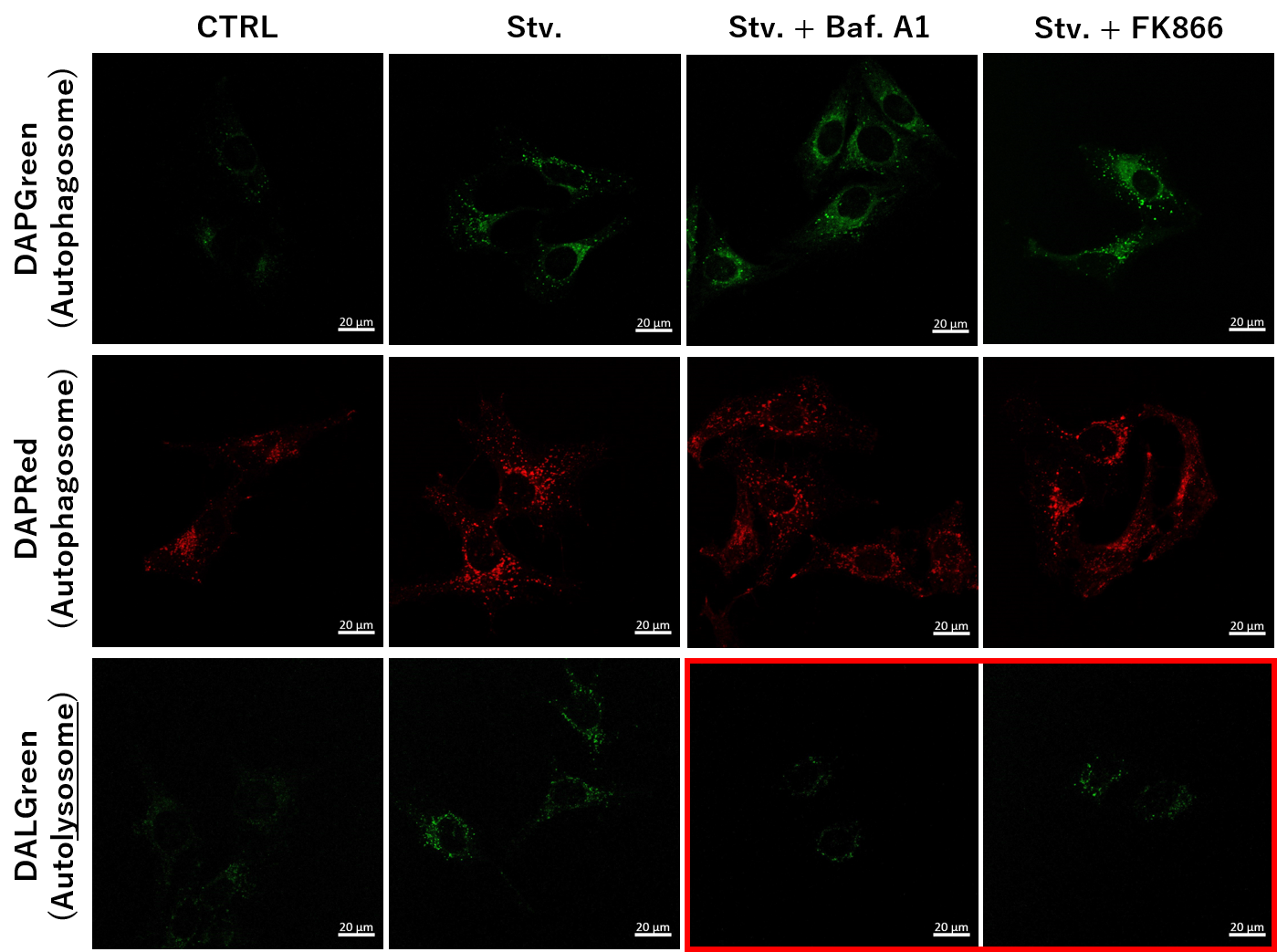
We next determined how FK866-induced lysosomal deacidification affects the autophagy-lysosomal pathway. After staining with DAPGreen/DAPRed (for detecting autophagosome), or DALGreen (for detecting autolysosome), HeLa cells were starved in HBSS incubation and then treated with FK866 or Bafilomycin A1. Under the starvation condition, the fluorescent signals from all dyes increased, indicating the proceeding autophagy-lysosomal pathway. On the other hand, only DALGreen's signals were decreased in FK866 and Bafilomycin A1 treated cells with starvation conditions. These results clearly suggested that FK866 inhibits the autophagy-lysosomal pathway by NAD+ depletion-induced lysosomal deacidification.
(Protocol)
HeLa cells (8 well ibidi) (MEM, FBS+)
↓
Single-stain with 2 umol/I DALGreen or
0.2 umol/I DAPGreen or
0.2 umol/I DAPRed
30 min, 37℃
↓
Wash x2 (MEM, FBS+)
↓
. Control (MEM, FBS+) for 2 h 20 min
· Starvation (HBSS) for 2 h 20 min
· Stv.2 h = 10 nM Bafilomycin A1 (HBSS) for 20 min
· Stv.2 h = 10 nM FK866 (HBSS) for 20 min
Observed by confocal microscopy (x40)
|

















