|
Autophagy is a cellular process that degrades and recycles damaged cellular components and is critical for maintaining cellular health and function. In neurons, autophagy plays an important protective role by removing defective proteins and organelles, preventing the accumulation of toxic aggregates that can lead to neurodegenerative diseases. This process also helps maintain synaptic plasticity and neuronal homeostasis, which are essential for proper neuronal function and brain health. In addition, by regulating the response to neural stress, autophagy contributes to the overall resilience and longevity of neural cells.
|
-
The aging mouse CNS is protected by an autophagy-dependent microglia population promoted by IL-34
Click here for the original article: Rasmus Berglund, et. al., Nature Communications, 2024.
Point of Interest
- A microglial population emerges in the cortical regions of aging mice, characterized by a transcriptome indicative of activated autophagy.
- This population is found to be dependent on IL-34, a ligand for CSF1R.
- Loss of autophagy-dependent microglia leads to neuronal and glial cell death and increased mortality in aged mice exposed to neuroinflammation.
- Conversely, IL-34-mediated microglial expansion is protective.
-
Lysophagy protects against propagation of α-synuclein aggregation through ruptured lysosomal vesicles
Click here for the original article: Keita Kakuda, et. al., PNAS, 2023.
Point of Interest
- αSyn aggregates accumulate predominantly in lysosomes, causing them to rupture.
- This rupture seeded the aggregation of endogenous αSyn, initially around damaged lysosomes.
- Exogenous αSyn aggregates induced the accumulation of LC3 on lysosomes, indicating the activation of lysophagy.
- Autophagy-deficient cells treated with αSyn aggregates had an increased number of ruptured lysosomes and enhanced propagation of αSyn aggregates.
-
The Hippo pathway noncanonically drives autophagy and cell survival in response to energy stress
Click here for the original article: Gayoung Seo, et. al., Molecular Cell, 2023.
Point of Interest
- Hippo kinase MAP4K2 is essential for autophagy and cell survival under energy stress.
- MAP4K2 phosphorylates LC3A at Serine 87 to promote autophagic flux.
- Energy stress induces the formation of the MAP4K2-STRIPAKSTRN4 complex, activating MAP4K2.
- Autophagy mediated by MAP4K2 is critical for the development of head and neck cancer.
|
|
Related Technique in This Topic
|
- Autophagy detection
- DAPGreen / DAPRed (Autophagosome detection), DALGreen (Autolysosome detection)
|
- Lysosomal function assay
- Lysosomal Acidic pH Detection Kit-Green/Red and Green/Deep Red
|
- Endocytosis detection
- ECGreen-Endocytosis Detection
|
|
|
|
|
- Mitophagy Detection
- Mitophagy Detection Kit and Mtphagy Dye
|
- Extracellular vesicles labeling
- ExoSparkler Exosome Membrane Labeling Kit-Green, Red, Deep Red
|
- Total ROS detection
- Highly sensitive DCFH-DA or Photo-oxidation Resistant DCFH-DA
|
- Mitochondrial superoxide detection
- MitoBright ROS Deep Red - Mitochondrial Superoxide Detection
|
- Antibody/Protein labeling with fast and high recovery
- Fluorescein, Biotin, and Peroxidase Labeling Kit - NH2
|
Tracing autophagosome to autolysosome in live cells
Nampt inhibitor, FK866 inhibits the progress of autophagosome to autolysosome by lysosomal deacidification. A recent finding shows that the dysfunctional condition of nicotinamide adenine dinucleotide (NAD+) biosynthetic enzyme, Nampt induces lysosomal deacidification1). In this section, we tried to determine how NAD+ depletion-induced lysosomal deacidification affects the autophagy-lysosomal pathway. 1) Mikako Yagi, et. al., EMBO J., 40(8), e105268 (2021)
-
<Impact on Lysosomal Acidification and pH Detection>
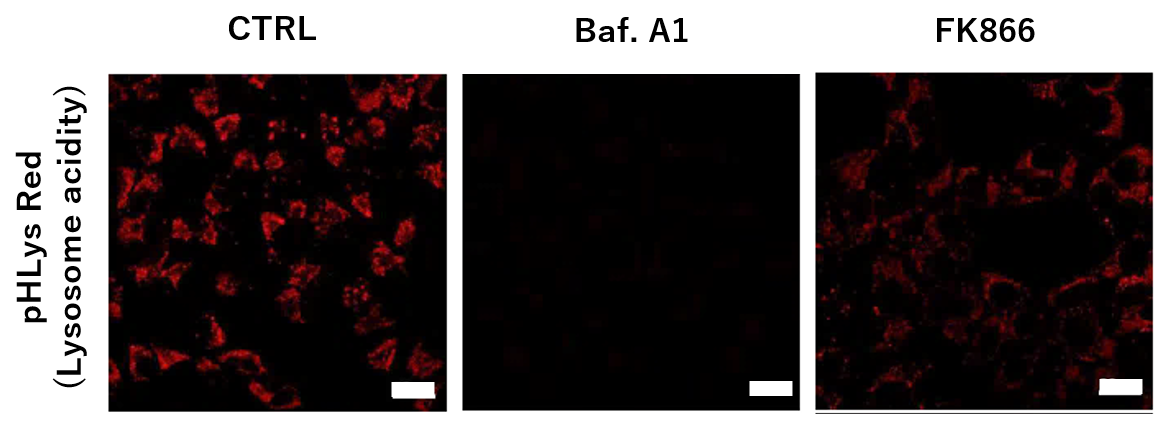
-
To confirm the effect of the Nampt inhibitor, FK866, on lysosomal acidification, HeLa cells were first labeled by the lysosomal pH detection dye pHLys Red. The cells were then treated with FK866, and lysosomal acidification inhibitor Bafilomycin A1 was used as a positive control. FK866 and Bafilomycin A1-treatment each decreased the fluorescent pHLys Red signal, indicating lysosome neutralization.
(Protocol)
HeLa cells (8 well ibidi) (MEM, FBS+)
↓
Wash x2 (HBSS)
↓
. pHLys Red working solution (HBSS, 1,000 times dilution)
. pHLys Red working solution (HBSS, 1,000 times dilution) + 50 nM Bafilomycin
A1
. pHLys Red working solution (HBSS, 1,000 times dilution) + 10 nM FK866
↓
30 min, 37°C
↓
Observed by confocal microscopy (x20)
|
-
<NAD+ Depletion and Autophagy-Lysosomal Pathway Response>
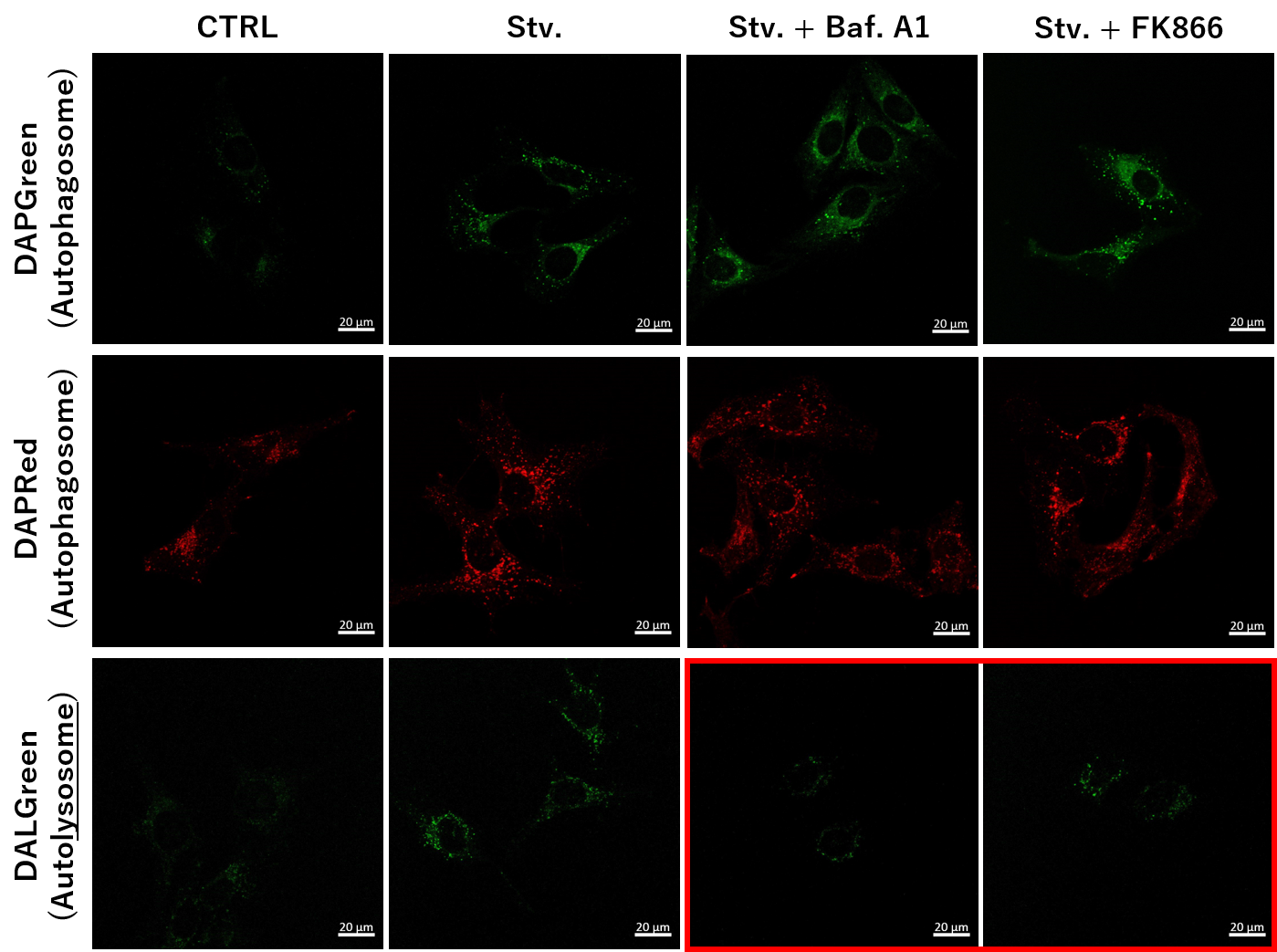
-
We next determined how FK866-induced lysosomal deacidification affects the autophagy-lysosomal pathway. After staining with DAPGreen/DAPRed (for detecting autophagosome), or DALGreen (for detecting autolysosome), HeLa cells were starved in HBSS incubation and then treated with FK866 or Bafilomycin A1. Under the starvation condition, the fluorescent signals from all dyes increased, indicating the proceeding autophagy-lysosomal pathway. On the other hand, only DALGreen's signals were decreased in FK866 and Bafilomycin A1 treated cells with starvation conditions. These results clearly suggested that FK866 inhibits the autophagy-lysosomal pathway by NAD+ depletion-induced lysosomal deacidification.
(Protocol)
HeLa cells (8 well ibidi) (MEM, FBS+)
↓
Single-stain with 2 umol/I DALGreen or
0.2 umol/I DAPGreen or
0.2 umol/I DAPRed
30 min, 37℃
↓
Wash x2 (MEM, FBS+)
↓
. Control (MEM, FBS+) for 2 h 20 min
· Starvation (HBSS) for 2 h 20 min
· Stv.2 h = 10 nM Bafilomycin A1 (HBSS) for 20 min
· Stv.2 h = 10 nM FK866 (HBSS) for 20 min
↓
Observed by confocal microscopy (x40)
|

















